From the laboratory to the patient, CaixaImpulse succes stories
08.07.25
4 minutes readSend your questions to:
The CaixaImpulse call for proposals to support health innovation projects has been bringing research closer to patients for a decade. Here we highlight some of the 232 biomedical and technological research projects supported by the programme in Spain and Portugal that have reached advanced stages of development or made it to market.
Time is Brain
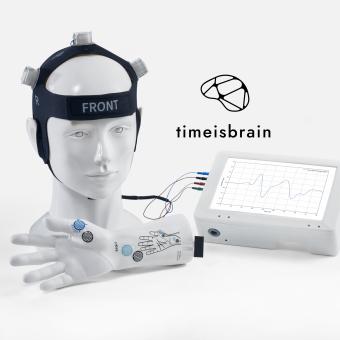
The spin-off from Hospital Germans Trias i Pujol, Time is Brain, has developed BraiN20®, a portable medical device that allows real-time monitoring of brain viability after a stroke. It processes a biomarker called N20 and displays the brain’s condition in real time, providing key information to assess prognosis and speed up medical decisions.

It is currently in the validation phase across seven Spanish hospitals and is close to obtaining CE marking – an essential step for commercialisation in Europe. With public and private backing already exceeding nine million euros, Time is Brain aims to complete clinical trials in 2025, launch its first product to market and expand its technology to address other neurological conditions.
Inrobics

Another project supported by CaixaImpulse, Inrobics, focuses on social robotics and artificial intelligence applied to physical and cognitive rehabilitation, as well as the care of older adults, with the aim of improving their quality of life.
Founded as a spin-off from the Carlos III University of Madrid and led by its CEO José Carlos Pulido, the company has made significant advances, such as improving motor learning in patients with spinal cord injuries following sessions with its robot, Robic.
Pulido highlights that CaixaImpulse helped them professionalise the project and connect with the healthcare system. The result is a human-centred and clinically validated approach, with which Inrobics is committed to delivering accessible and effective technology. The company has raised two million euros and has opened a new funding round.
Innitius
CaixaImpulse has also supported Innitius, a spin-off from the University of Granada and the Andalusian Health Service, which is set to revolutionise maternal and foetal health with CerviSense, a medical device that uses artificial intelligence and torsional wave technology to improve early diagnosis and personalised care in preterm births and labour induction.
The project, led by engineer Rubén Molina, has successfully completed a clinical evaluation in ten Spanish hospitals, including Vall d’Hebron, Hospital Clínic and Sant Joan de Déu in Barcelona.

Innitius has received support from the CaixaImpulse programme, which has been key to advancing the clinical validation of the device and preparing it for future commercialisation, backed by ten million euros in public and private funding.
Other companies with
significant social impact
In addition to these cases, which shared their experiences at a media event held at the Palau Macaya in Barcelona, other innovative projects that have taken part in the CaixaImpulse programme stand out for their strong impact on patients’ health.
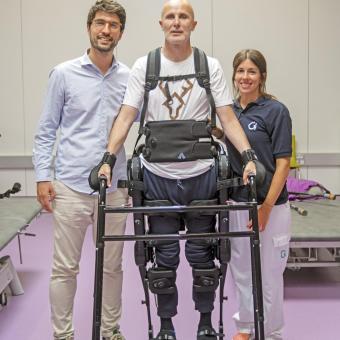
One such project is ABLE Human Motion, which, under the leadership of engineer Alfons Carnicero, has democratised lightweight exoskeleton technology to improve mobility and independence for wheelchair users who have suffered a spinal cord injury, a stroke, or who live with multiple sclerosis.
In 2025, they launched the first 15 rehabilitation devices in private clinics in Spain. Soon, their exoskeleton will be introduced into the public healthcare system. They are now looking to expand into other countries, while working on a different device for home use.

Another example is Arthex Biotech, a spin-off from the University of Valencia co-founded by Beatriz Llamusí and Rubén Artero, which has developed an RNA-based therapy for myotonic dystrophy. This rare degenerative disease causes severe multisystemic problems, with symptoms affecting the nervous, musculoskeletal and cardiac systems.
After five years, they have succeeded in bringing the product to the clinical phase. By the end of 2026, they will complete the clinical trial and obtain data on safety and whether the mechanism of action of their therapy works. To date, they have secured 60 million euros in public and private funding.

Another good example is Corify, spin off from the Hospital Gregorio Marañón that has developed a non-invasive cardiac mapping technology to improve arrhythmia diagnosis, and it has recently been included in the Clinical Guidelines of the European Society of Cardiology (ESC).
Meanwhile, Gate2Brain is working on a platform that facilitates drug delivery to the brain for the treatment of paediatric tumours and has received Orphan Drug Designation (ODD) from both the European Medicines Agency (EMA) and the United States Food and Drug Administration (FDA) for medicines aimed at treating rare diseases.
At the same time, Aortyx is developing bioabsorbable patch technology to reduce mortality rates caused by aortic dissection and has recently secured a 13.8 million euro funding round.