You are reading:

You are reading:

Regenerating skin or delivering medication to specific areas of our body is now possible thanks to the inspiration scientists draw from nature. Three projects supported by the CaixaResearch calls for Research and Innovation in Health proposals are exploring whether animals such as mussels, zebrafish or bees hold some of the keys to treating diseases that pose a challenge to the international medical community.
Over billions of years of evolution, nature has developed solutions that enable living beings to adapt to their environment and survive. Can humans learn from those with proven experience in creating innovative and efficient solutions? That is precisely what biomimicry does – a discipline focused on improving human life by studying and replicating biological structures and their functions.
The fact is that many of the answers to the challenges we have today could be found by looking to nature, just as Leonardo da Vinci did when he drew inspiration from birds to design flying machines, or Alexander Fleming, whose discovery of penicillin came from observing how a fungus inhibited bacterial growth – leading to the development of antibiotics and revolutionising medicine.

In the biomedical field, biomimicry has had a significant impact in recent years, achieving important advances for human health. Scientists have succeeded in developing biocompatible materials, made great progress in regenerative medicine and even enabled certain drugs to reach specific areas of the body for targeted treatment.
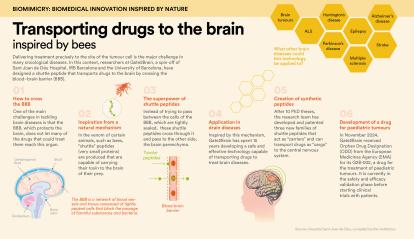
Delivering treatment precisely to the location of tumour cells is a major challenge in many oncological diseases. In this regard, researchers from Gate2Brain, a spin-off of Sant Joan de Déu Hospital, the IRB Barcelona and the University of Barcelona, have designed a peptide carrier capable of transporting drugs to the brain and crossing the blood-brain barrier (BBB). This technology will enhance the treatment of brain diseases, including paediatric brain cancer, by optimising drug delivery to this organ.
While several drugs had previously demonstrated strong antitumour activity, all had failed in clinical trials because they could not cross the BBB to reach the tumour. “It’s a protective cellular barrier that only allows certain molecules from the circulating blood to enter the central nervous system, in order to prevent the entry of pathogens and other harmful substances,” explains Dr Meritxell Teixidó, CEO and co-founder of Gate2Brain. The company collaborates with the Childhood Cancer Research Unit – ”la Caixa” Foundation at Sant Joan de Déu Hospital.

The research team led by Teixidó –which received support from RecerCaixa, CaixaImpulse Validate and CaixaImpulse Consolidate– studied the potential of peptides found in scorpion and bee venom to function as molecular shuttles. “We were looking for peptides, which are very small proteins, that could act as molecular shuttles to transport drugs to the brain. We found that bees, like many other animals, produce a shuttle peptide in their venom that carries its toxin to the central nervous system, but which could also be used to deliver drugs for treatment,” she explains. “These shuttles don’t just pass between the BBB cells, which are tightly sealed, but rather enter them and exit on the other side into the brain parenchyma,” she adds.
In general, improving drug delivery to the brain brings significant benefits for the patient, as administering the precise dose enhances treatment effectiveness and reduces side effects.

Moreover, the results obtained have the potential to be applied to other therapeutic agents and biological barriers, with significant clinical implications. “There are many drug candidates for brain diseases, but 98% will require some kind of shuttle. Our technology could be applied to many of them,” confirms the researcher.
In November 2024, Gate2Brain reached a decisive milestone in the fight against paediatric brain tumours when it received Orphan Drug Designation (ODD) from the European Medicines Agency (EMA) for its innovative product, G2B-002. The project is currently at the regulatory preclinical stage, transitioning from trials in rodents to non-rodents (specifically pigs). “Although the clinical trial is planned for three years from now, we’re at a highly significant moment for this technology and our first product,” adds Dr Teixidó.
Her team is working tirelessly to usher in a new era of therapeutic options in an area of high unmet need. “In recent years, great progress has been made in diagnosing brain tumours, in developing more advanced therapies, but most importantly, in understanding these tumours to enable much more personalised medicine. Shuttles could be the key ingredient that helps these therapies reach their target more effectively,” she concludes.
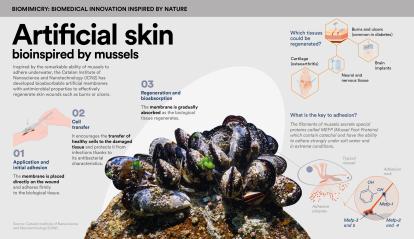
The creation of artificial skin is a top medical priority. The healing process of skin wounds, such as burns or ulcers (which are very common in conditions like diabetes), is slow and complex. Unfortunately, in many cases, it is hindered or even completely blocked due to the lack of effective treatments.
The team led by Salvio Suárez, a researcher at the Catalan Institute of Nanoscience and Nanotechnology (ICN2), has developed a family of artificial membranes bioinspired by the ability of mussels to adhere underwater. These membranes have already been validated in previous preclinical studies for cartilage and skin regeneration. The new materials adhere to biological tissue, are bioabsorbable, have antimicrobial properties and can transfer cells to damaged tissue, thereby promoting its regeneration.

As Suárez explains, “We initially focused on cartilage and found that the regeneration was very positive, which led us to explore skin regeneration, as it allowed us to study the membrane on a broader scale.” They then conducted a second preclinical study on the regeneration of common wounds and observed that they could reduce the healing time by up to half. Currently, they are engaged in a second preclinical trial on pigs, “as the characteristics of the skin of these animals are more similar to those of human skin”.

This project, supported by the CaixaImpulse Health Innovation call, aims to optimise and validate artificial membranes with both regenerative and antibacterial properties. “The antimicrobial properties are derived from the molecules found in mussels, which served as our inspiration,” explains the materials science expert. Their advantage lies in their ability to oxidise over time. “The membranes we produce, by being bioinspired by these molecules, also undergo oxidation and generate chemical species such as hydrogen peroxide, which has disinfectant properties.” Furthermore, this antimicrobial mechanism has not yet led to bacterial resistance. “Therefore,” says Suárez, “we have a broad spectrum of action, even against multi-resistant bacteria.”
This disruptive technique can shorten hospital stays, reduce infections – which are very common in burn patients – and ultimately minimise graft rejection. “What we aim for,” the researcher notes, “is for the new skin to resemble the original as closely as possible. From a functional perspective, we’ve managed to achieve properties of flexibility, permeability and sensitivity that are very similar.”
The researchers are also considering extending its potential use to the treatment of other diseases. “We’re validating its properties for neuronal regeneration and also for the treatment of certain types of tumours,” he says.
The ultimate goal is to carry out a first clinical trial in humans. “We expect to begin patient recruitment in the second half of 2026,” he concludes. Additionally, the researchers have recently founded the company Tirecat Health SL to further develop this technology and explore other applications.
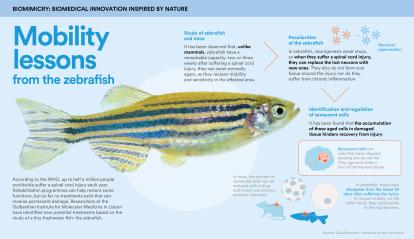
According to the World Health Organization (WHO), up to half a million people worldwide suffer a spinal cord injury each year. Rehabilitation programmes can help restore some functions and reduce the risk of complications, but so far, no treatments exist that can reverse permanent damage.
Recently, a project supported by the CaixaResearch Health call in collaboration with the Fundação para a Ciência e a Tecnologia (FCT) has identified new potential treatments.

Leonor Saúde, a researcher at the Gulbenkian Institute for Molecular Medicine in Lisbon, specialises in developmental biology and has extensively studied the embryonic processes of the zebrafish. She and her team observed a remarkable ability in this tiny freshwater fish: within two to three weeks of suffering a spinal cord injury, it resumes swimming normally, having regained mobility and sensation in the affected area. In contrast, mammals such as mice typically show very little improvement within the same timeframe. “We saw an opportunity to gain new insights that might one day be applied to patients with spinal cord injuries,” she explains.
Saúde’s team conducted a thorough investigation of both animals to determine the cause of such starkly different outcomes. They observed that zebrafish, which never stop growing, continuously generate new neurons. As a result, if they suffer a spinal cord injury they can replace lost neurons with new ones. “They also don’t form scar tissue around the injury or experience chronic inflammation,” explains the expert.
However, the team’s biggest surprise was “the stark difference in the number of senescent cells in each model”, reveals Saúde. “These are cells that accumulate with age in any tissue of the body. So in a way, senescence is an indicator of ageing. In zebrafish, these cells disappear from the tissue 30 days after the injury. In contrast, in mouse models, they continue to accumulate in the injured area.”
The research team decided to use a senolytic drug to reduce the number of aged cells in mice with spinal cord injuries and discovered that motor and sensory recovery improved. “We concluded that these cells have a negative effect on spinal cord injuries: they significantly hinder recovery because they secrete inflammatory and profibrotic molecules, which contribute to inflammation and scar formation,” she explains.
“Currently, we’re conducting single-cell RNA sequencing experiments to better characterise these cells and their secretory phenotype [the set of molecules they secrete] at specific time points after an injury,” adds the expert. Their goal is to understand what type of cells they were before becoming senescent, identify the molecules they produce and design more precise strategies to manipulate these factors and reduce their negative impact on tissues.
In the future, the researcher predicts that, given the complexity of this medical condition, the treatment of such injuries will likely combine therapies such as neuroprotection, neurostimulation, stem cell transplantation and perhaps others that are yet to be discovered.
Collaboration has always proven to be a key driver of scientific progress, but it is not the only one. As demonstrated by these three studies, nature also plays a fundamental role – and will continue to do so. In fact, “just three years ago, it was discovered that a mammal – the Acomys or spiny mouse – unlike others, was capable of regenerating its spinal cord,” Saúde reveals. “The reason why is still being investigated, but it’s clear that we scientists must continue looking to nature to learn from it,” she concludes.