
You are reading:

You are reading:

16.05.24
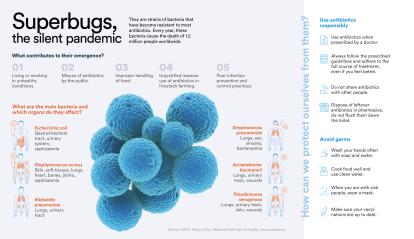
9 minutes readEvery year, 1.2 million people around the world die from infections caused by antibiotic-resistant bacteria. According to the WHO, the ability of bacteria to adapt and resist drugs designed to combat them is already one of the biggest threats to global public health. If left unchecked, antimicrobial resistance could cause up to 10 million deaths worldwide by 2050. Four research projects funded by the 2023 CaixaResearch Health and CaixaImpulse Health Innovation calls seek to tackle this major challenge for humanity with very different approaches.
When Daniel López Serrano, a biochemist at the National Centre for Biotechnology of the Spanish National Research Council, started doing basic research in a laboratory in the early 2000s and requested samples from hospitals, he found only one superbug in the 20 samples he received. Today, it is not uncommon for all 20 to contain antibiotic-resistant bacteria. That is why discovering a new, effective antibiotic is such a challenge for the pharmaceutical industry. “Research can take decades with no guarantee of results, and drug resistance is appearing faster and faster, which discourages investment in the research and development of new antimicrobials,” says López Serrano.

The project he is leading aims to restore the effectiveness of antibiotics that have become ineffective due to the increase in resistant bacteria. In 2010, his team discovered a cellular process that is essential for bacteria to survive during infections. He explains: “This mechanism is responsible for stabilising and assembling the protein machinery within the bacteria, including the machinery responsible for antibiotic resistance. Over the years, we’ve focused on understanding this mechanism and have been able to develop treatments that inhibit it. This prevents the assembly of all that machinery.” In this situation, the administration of classic antibiotics works, because the bacteria have deactivated their resistance mechanisms.
According to López Serrano, the great advantage of this project, which has received a grant of 494,300 euros from the 2023 CaixaResearch Health call for proposals, is precisely that it proposes a treatment capable of curing infections that previously could not be cured, by reactivating commercially available antibiotics.
“What we’ve discovered is not a molecule, it’s a mechanism, so the most direct way to implement it is probably to develop it as a process or a hospital therapy, rather than commercialise it,” argues the researcher. “So far, we’ve designed the procedure for a couple of bacterial species and we’ll try to extend this knowledge to other species, the most common ones in hospitals, to create a treatment that’s universal.”
When a new antibiotic is developed, it may have the ability to attack bacteria but not be able to cross the bacterial membrane. Current strategies to facilitate this membrane crossing have not been entirely effective. The project led by Javier Montenegro, a chemist at the Center for Research in Biological Chemistry and Molecular Materials (CiQUS) of the University of Santiago de Compostela, in collaboration with Mariana Pinho from the Nova University Lisbon, uses a new approach to transporting antibiotics into bacteria that has great potential to enable the use of existing antibiotics in strains that are resistant or have limited permeability.

“This is a very complex challenge because it’s not easy to cross the bacterial membrane, especially Gram-negative bacteria, which have an outer membrane that poses an additional barrier,” explains Montenegro. In many cases, current approaches “use lipids to cross membranes formed by other lipids,” which requires “the encapsulation of the molecules, which can affect both their stability and the release of the antibiotic at the right time,” he adds.
However, Montenegro and Pinho’s team have discovered a property of boron clusters that allows hydrophilic molecules – molecules that dissolve in water – to pass through lipid membranes. For Montenegro, this major scientific milestone, which has received 984,050 euros from the CaixaResearch Health Call 2023, represents “a complete paradigm shift for science and drug delivery. It’s very novel research and completely different from the established dogma. Now we need to understand the basis of this mechanism so that we can apply it to the transport of antibiotics.”
Although antibiotics can eliminate micro-organisms, paradoxically they also contribute to the emergence of resistant bacteria and facilitate their proliferation in an environment free of competitors. Moreover, antibiotic development is currently stagnating.
For this reason, some research projects are focusing on developing alternative therapeutic strategies to deactivate the virulence of superbugs and re-sensitise them to antibiotics, while preserving the microbiota, the natural community of bacteria in our bodies that can be altered by regular antibiotic treatment. This delicate ecosystem plays an essential role in our intestinal, dermatological and mental health, as well as in our immune system, our defence against pathogens, our metabolism and the regulation of our body weight.

The team led by Didier Cabanes, a molecular biologist at the Institute for Research and Innovation in Health of the University of Porto, is studying Gram-positive bacteria such as staphylococci, streptococci and Listeria. In the cell wall of Listeria, they found that “some glycopolymers (sugars) present on its surface – which were not essential for its growth – were crucial not only for the bacterium’s resistance to host antimicrobial defences and antibiotics, but also for its pathogenicity”. Consequently, the researchers believe that “the enzymes responsible for the presence of these glycopolymers on the bacterial surface are promising targets for antimicrobial drugs against Gram-positive pathogens”.
The project, in collaboration with the Fundação para a Ciência e a Tecnologia (FCT), has received a grant of 499,950 euros from the 2023 CaixaResearch Health call. Its ultimate goal is to develop “next generation” antimicrobial drugs which, by inhibiting the glycopolymers present on the surface of superbugs, reduce their virulence without killing them. This will gradually render them vulnerable to the host’s defences, enhancing the effect of antibiotics while preserving the microbiota. “Our approach is the first to simultaneously disarm and sensitise Gram-positive pathogens without affecting their growth,” concludes Cabanes.
The latest report from the European Centre for Disease Prevention and Control includes Klebsiella pneumoniae among the top three causes of antibiotic-resistant infections. Specifically, more than 45,000 cases of infection caused by this bacterium were detected in Europe in 2022, over 15% of which were caused by strains immune to the effects of these drugs. This is why the WHO has classified Klebsiella pneumoniae as a species of critical health concern and why the team led by Mireia López Siles, a microbiologist at the Universitat de Girona, decided to develop a vaccine to continue the fight against this bacterium.
Klebsiella pneumoniae can be found in any environment. In a hospital setting, it is usually spread by contact with the skin, mucous membranes, faeces, wounds or urine of an infected person. The situation is made worse if the strain causing the infection is also resistant to antibiotics. A vaccine could extend the duration of protection against the bacteria compared to antibiotic treatment, provide herd immunity and reduce the mortality associated with these infections. “This is crucial because it would reduce the use of antibiotics to treat infections and the likelihood of more antibiotic-resistant bacteria appearing,” explains López Siles.

The project, which has received 50,000 euros from the CaixaImpulse Health Innovation call (funding supplemented by the support of experts and mentors), is in the preclinical phase. The aim in this phase is to evaluate the in vivo protective capacity of KlebsiGene, a DNA vaccine previously developed by the Universitat de Girona and the Instituto de Salud Carlos III, and to improve its formulation. It includes two main innovations, according to the researcher: “On the one hand, the vaccine targets parts of the bacterium that have not been previously used in other vaccines and that play a key role in its survival, so we expect it to provide better protection. On the other hand, we’re using a new DNA technology that incorporates elements to stimulate the patient’s immune response.”
So far, López Siles’ team has developed eight vaccine prototypes and found that human cells correctly interpret the information provided by these vaccines. “We’ve also seen, through prediction using bioinformatics tools, that not all vaccine candidates would be equally effective in generating an immune response. This has enabled us to prioritise the most promising prototypes for efficacy testing. We’ve just completed the production phase of two of these. The next step is to demonstrate in a complex organism that our vaccine is capable of activating the immune system and providing protection against infection.”
The development of this vaccine “will improve the quality of life for people at risk of infection by this micro-organism, especially in the hospital setting,” adds the researcher. Furthermore, being a DNA vaccine, it is easily adaptable to other pathogens, “because it contains a specific DNA fragment that encodes for the antigen (the part that allows the immune response to develop), which in our case is specific to Klebsiella pneumoniae. By replacing this fragment with one encoding the antigen of any other microorganism, we would obtain a vaccine targeting that other species. And it could also be designed to prevent infections by several pathogens simultaneously, if antigen sequences for both are included.”
This widespread increase in superbugs “is partly natural – bacteria evolve and acquire resistance,” explains López Serrano. “But with our use of antibiotics we’ve also contributed to creating environments, such as hospitals or livestock farming (where antibiotics have been widely used, not only to treat bacterial infections in animals but also to promote their growth and prevent disease without prior diagnosis), that facilitate this evolutionary process,” making antibiotics increasingly ineffective.
For some time now, health authorities have been working on strategies to rationalise the use of antibiotic drugs, including that of raising public awareness. As Didier Cabanes reminds us, we should use them “only on medical prescription and only when really necessary”. “Treatments should be completed,” adds López Serrano, “and not kept around to be taken whenever we want or given to our neighbours. Another important aspect is the disposal of antibiotics: if we flush leftover antibiotics down the toilet, they end up in the environment and the bacteria are there too, so it’s very important to dispose of them in the collection points set up in pharmacies.” The future of superbugs is also in our hands.
To learn more about superbugs, you can watch the CaixaResearch debate on this topic: