
You are reading:

You are reading:

03.05.24
9 minutes readKaori Mutsuda Zapater (Barcelona, 1996) is a biotechnologist. Thanks to a postgraduate fellowship abroad from the ”la Caixa” Foundation, she is studying for her PhD in Kyoto, in a new faculty dedicated to finding solutions to ensure human survival. Her specialisation is organs-on-a-chip, miniature devices that create simplified versions of body parts. Her research aims to recreate one of the most important and lesser-known processes at the beginning of life: the implantation of a fertilised ovum in the uterus. Prior to all this, she had a long career in film dubbing and, among other roles, provided the voice for the little girl in Monsters, Inc.
You do research at an institute that focuses on the survival of humanity. What are these studies?
My faculty is the newest at Kyoto University, and it offers Advanced Integrated Studies in Human Survivability. It’s a five-year programme that brings together people from different fields – literature, philosophy, medicine, engineering, etc. – to work together to address global challenges. The idea is to add leadership skills and basic concepts from other disciplines to the specialisation: we have classes from other sectors, seminars with industry professionals and compulsory stays abroad that allow us to incorporate this global perspective into our research.
And the main objective is to prevent the end of humanity?
Actually, the approach is quite open. This faculty is interested in any topic that makes sense in terms of human survival, but some people are working on the intersection of Buddhist and Greek philosophy, for instance; others are researching geopolitics and international relations..... In my case, I work in health sciences, in the field of reproduction, so it’s very much related to the preservation of life.
How do they see the future? Should we be optimistic?
At the faculty level, my sense is that this is an area of optimistic and positive of research; but on an individual level, there are all kinds of opinions. Some people are researching for catastrophic scenarios, focusing on basic survival; others are studying at the possibilities of colonising Mars and living in space, for example. There are very diverse approaches.
Did you choose to study in Japan because you were attracted to the subject?
I applied twice to the ”la Caixa” Foundation postgraduate fellowship abroad programme and, in fact, initially opted for Cambridge, but I wasn’t chosen, so I decided to apply again the following year. I finally went for a position at Kyoto University because I was very interested in the research group: it’s not very common to find groups that combine disciplines like cell culture, especially pluripotent cell culture, and microfluidics. What’s more, Kyoto University has a lot of resources and expertise in the use of pluripotent cells (Shinya Yamanaka won the 2012 Nobel Prize in Medicine for his research in the field of stem cells).
Where do your Japanese roots come from?
My father’s Japanese and my mother Catalan. He came to Spain to study when he was about 20 and stayed. I was born and raised in Barcelona and went to a Catalan school first, and then to a Japanese school. I did all my compulsory education there and then I studied at the University of Barcelona. So I’ve actually spent my whole life in Spain.
After studying biotechnology you started to work in a pharmaceutical company, but you left. Why?
When I finished my degree, I did a Master’s in Business Administration (MBA), which was focused on the pharmaceutical industry and included an internship in a company. I’ve always wanted to be an entrepreneur or find a way to transfer scientific value to society, so that it doesn’t just stay in academic publications. That’s why I wanted to see how the pharmaceutical sector works from the inside. I really enjoyed the experience, but I wanted to explore other fields.
What discipline does your work fall under?
The technical term is tissue engineering. We take cells, in my case human cells, and create tissues and simplified versions of organs that allow you to understand how a disease develops and how it responds to different stimuli or drugs, among other things. We use a technology called “organ on a chip", which lets you recreate organs or parts of the body (the cornea, the heart, the brain, etc.) in small devices that are connected to each other by little plastic tubes, like veins. It’s not a miniature version of a real organ, but a mechanism that brings together its key biological elements.
Could these chips be put together to create a complete human being?
One of the long-term objectives is to recreate the human body on a chip. Not just one human body, but every human body, for personalised medicine applications. At the moment it’s not entirely realistic because it’s technically very complex. There are also difficult ethical issues that need to be addressed. A more realistic goal is to simulate specific phenomena or diseases. If you want to study diabetes, for example, you can attach a pancreas chip and a muscle chip without having to represent a whole human body.
What is the advantage of doing it on such a small scale?
It’s a model that can be more cost-effective in the long run and also more representative. For example, instead of recruiting 50 people for a clinical trial, you have thousands of chips with organs of different characteristics and you obtain more meaningful data with fewer resources. It’s true that, right now, since the process is not very automated, it requires labour, which makes it more expensive, but in the future it could be much cheaper and more accurate than animal testing.
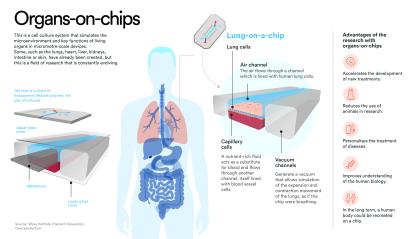
Is animal welfare also a reason?
My motivation is primarily ethical, I don’t like seeing animals suffer. Also, the vast majority of drugs tested on animals don’t make it to the market because they fail when tested on humans. Only 2% of those products are marketed and some are eventually withdrawn because of adverse effects. I’d like to create an alternative model that’s cheaper, ethical, inclusive and representative of what it means to be human.
What is your specific research project?
I specialise in the reproductive side; specifically, I want to recreate the process of human embryo implantation using artificial models of the uterus and the fertilised ovum. Instead of using fertilised ova from human donors, we thought, “Why not create an artificial reproduction of those early stages, prior to an embryo?”. It’s about manufacturing what’s called a blastocyst. We don’t use ova or sperm: we use pluripotent stem cells, either of embryonic origin or adult non-sexual cells that have been treated to return them to a primordial state. The interesting thing about these cells is that they have the ability to become anything you want them to be.
It sounds like a complex process. Do you encounter many difficulties?
There’s a lot of trial and error, because if you want to recreate the uterus on a chip you first have to think about each part of that uterus, as there are several layers and each one has different cell types and needs its own ecosystem. And then you might need some kind of mechanical stimulation that simulates muscles, for example. All of this adds to the complexity and means that you’re constantly correcting a lot of little things.
Given that it deals with life-related topics, it must have significant ethical implications. Is that so?
Japan is a very strict country in terms of scientific ethics, and depending on the type of cells you’re experimenting with, you have to go through rigorous protocols. To use cells of embryonic origin, even though they’re from much earlier stages than the embryo, you have to attend mandatory training and record the work sessions, then present them to the university every year. Pluripotent cells are easier to handle in ethical terms because they’re cells taken from an adult human.
Could you create artificial life?
If by creating “life” you mean “a living human being”, this is not technically possible at the moment. By developing an artificial blastocyst you might think that it could potentially become a baby, but it’s not viable because it’s a culture in a tube that cannot develop into a life. What’s more, there’s an international committee that sets very strict rules for manipulating pluripotent cells to create human embryo models: we’re obliged to stop the culture before certain molecular markers appear. On the other hand, growing any kind of embryo in vitro is not trivial, and getting them to develop outside the womb is very complicated. There’s a team in Israel that’s been able to grow a (natural) mouse embryo in a kind of artificial womb, but it’s not yet possible to replicate the whole process in vitro.
Finally, there’s a curious aspect of your career. You were a voice actor and can be heard as the little girl from Monsters, Inc. Did you ever think about continuing with that career?
Yes, I started when I was three years old because my elder brother was in the business. Twenty-five years ago, it was a very small sector that basically worked through contacts, and in my case I auditioned because it was suggested to me one day when I was with him. I worked as a voice actress until I was 18. I loved it, but it’s a world that requires a lot of dedication and availability. It would be nice to keep it as a parallel activity, but being in Japan makes it difficult. Well, and my talent isn’t stratospheric